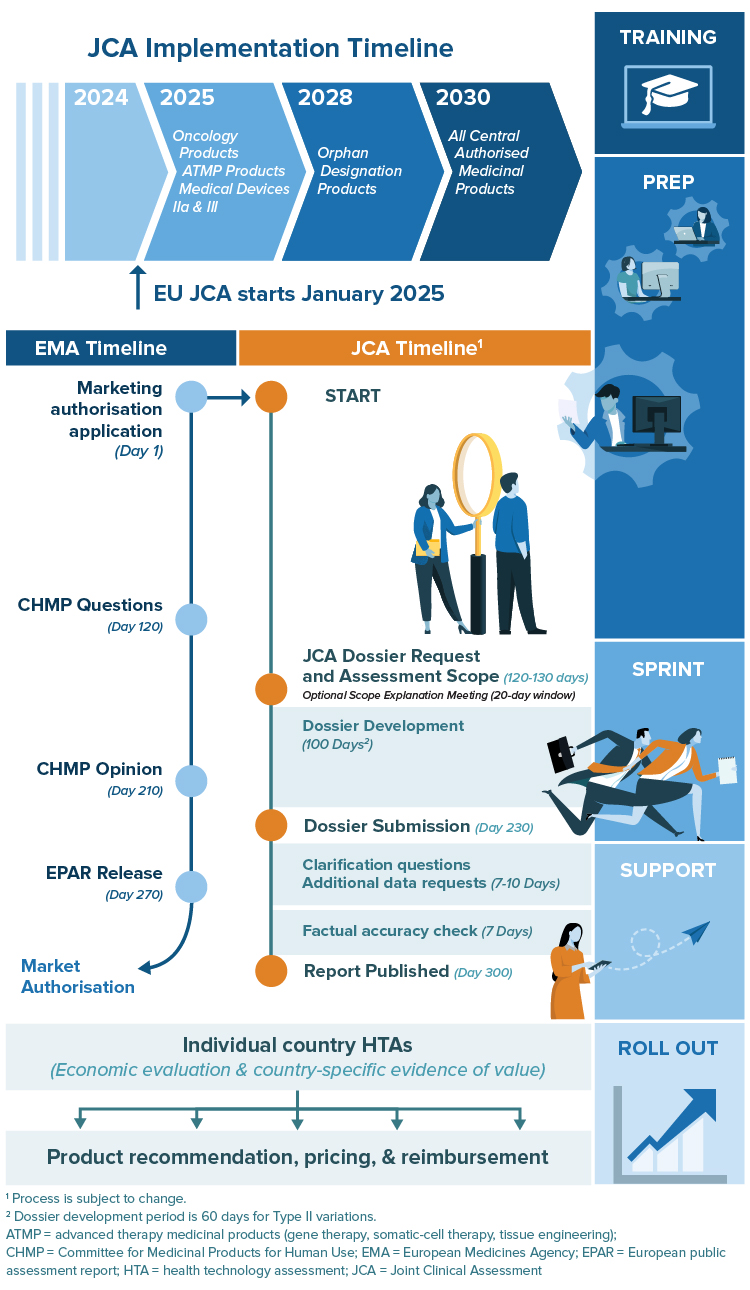
Get Ready for Your 100-Day Sprint
With only 100 days between confirmation of the Population, Intervention, Comparator(s) and Outcomes (PICOs) and dossier submission, companies getting prepared early will be in the best position for EU Joint Clinical Assessment.
RTI-HS can help you with advance preparation, developing, and refining your JCA dossier. As with our comprehensive support in preparation for global HTA, we can work to anticipate likely and appropriate PICOs, prepare the highest quality evidence in advance, and nimbly adjust to the final scope and label changes.