What do the joint clinical assessments (JCA) in Europe mean for your pharma or medtech company?
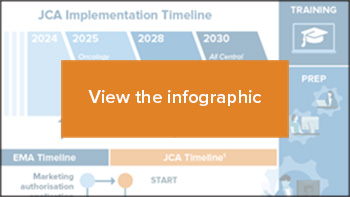
The parallel paths of the joint clinical assessment process and the marketing authorization process of the European Medicines Agency (EMA) place manufacturers in unsure territory.
The new regulation seeks to improve the availability of innovative health technologies across the EU.