Insights from Working with Newest AMCP Format Guidance During 2020
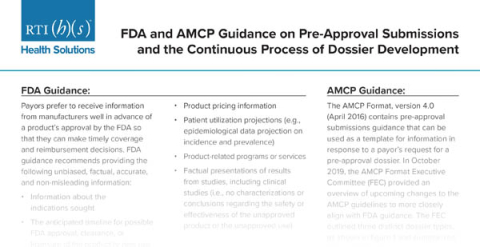
In December of 2019, the Academy of Managed Care Pharmacy (AMCP) released Version 4.1 of the AMCP Format for Formulary Submissions, which provides guidance for formulary submission dossier development. The updated guideline provides guidance to manufacturers regarding communicating clinical and economic evidence to support new products or new uses of existing products before gaining regulatory approval by the US Food and Drug Administration to benefit eventual market access.
Since the new guidance release, RTI Health Solutions (RTI-HS) has prepared numerous unapproved product dossiers (UPDs) and unapproved use dossiers (UUDs) in various therapeutic areas, including rare diseases, oncology, and women’s health. The purpose of this article is to share insights from our experiences developing these dossiers, with key considerations for manufacturers planning to develop early dossiers for their products.
Takeaway 1: Determine your internal stakeholders for pre-approval dossiers
I t is essential to determine key stakeholders and internal review committee members before beginning work on a UPD or UUD. It is critical to involve all stakeholders in preliminary discussions regarding content and plans for the dossier.
t is essential to determine key stakeholders and internal review committee members before beginning work on a UPD or UUD. It is critical to involve all stakeholders in preliminary discussions regarding content and plans for the dossier.
Takeaway 2: Decide on the objective for creating an unapproved product dossier or unapproved use dossier
 While this may seem straightforward, manufacturers can have different objectives for developing an early dossier. One manufacturer may consider it an opportunity to introduce the disease and unmet need, with minimal focus on ongoing or planned studies. Another may view it as an opportunity to present as much clinical data as early as possible. These objectives may also differ depending on the product or therapeutic area. In our experience, companies are more likely to develop a UPD as opposed to a UUD. This may be because manufacturers can include information on a potential new indication for an already approved product in the off-label section of an AMCP dossier for an approved product.
While this may seem straightforward, manufacturers can have different objectives for developing an early dossier. One manufacturer may consider it an opportunity to introduce the disease and unmet need, with minimal focus on ongoing or planned studies. Another may view it as an opportunity to present as much clinical data as early as possible. These objectives may also differ depending on the product or therapeutic area. In our experience, companies are more likely to develop a UPD as opposed to a UUD. This may be because manufacturers can include information on a potential new indication for an already approved product in the off-label section of an AMCP dossier for an approved product.
Takeaway 3: Decide on clinical data to be shared
 Considerable discussion among internal stakeholders regarding content is generally related to Section 3 of the AMCP dossier format, which contains clinical evidence in support of a product. All internal stakeholders should agree on the data to be made publicly available. There are often regulatory or internal legal constraints regarding the data that can be shared. There may be differences in marketing or launch strategies regarding which data can be discussed publicly before regulatory approval and launch. Manufacturers must determine the level of detail to provide, keeping the following considerations in mind:
Considerable discussion among internal stakeholders regarding content is generally related to Section 3 of the AMCP dossier format, which contains clinical evidence in support of a product. All internal stakeholders should agree on the data to be made publicly available. There are often regulatory or internal legal constraints regarding the data that can be shared. There may be differences in marketing or launch strategies regarding which data can be discussed publicly before regulatory approval and launch. Manufacturers must determine the level of detail to provide, keeping the following considerations in mind:
- What are the critical studies to be introduced in early discussions, and which data from these studies is essential to include?
- How will the inclusion of clinical data affect planned or pending publications?
Manufacturers should determine the appropriate approach for the release of specific clinical data early in the medical dossier development process to ensure that dossier development is not delayed.
A principal element of the decision-making process is determining the format for presentation of the data. Manufacturers may choose to present these data succinctly (as evidence tables) or more comprehensively (as study summaries and evidence tables).
Takeaway 4: Provide patient utilization projections
 While early dossiers will not contain economic models, manufacturers can include a description of the target patient population along with any patient utilization projections and patient funnel. The overall patient population can be described using epidemiological data. In cases where a product targets a specific patient population or subgroup, it may be useful to develop a funnel diagram to help the payer understand the size of the population to be treated. In some cases, a developer may feel that patient number projections are too preliminary to be included in the early dossiers.
While early dossiers will not contain economic models, manufacturers can include a description of the target patient population along with any patient utilization projections and patient funnel. The overall patient population can be described using epidemiological data. In cases where a product targets a specific patient population or subgroup, it may be useful to develop a funnel diagram to help the payer understand the size of the population to be treated. In some cases, a developer may feel that patient number projections are too preliminary to be included in the early dossiers.
Takeaway 5: Provide materials to develop the product information section
 Because the draft prescribing information is often unavailable during the development of a UPD or UUD, internal materials (such as the target product profile) should be considered to populate the relevant dossier sections. Such materials may contain information describing the product, its mechanism of action, and other essential attributes.
Because the draft prescribing information is often unavailable during the development of a UPD or UUD, internal materials (such as the target product profile) should be considered to populate the relevant dossier sections. Such materials may contain information describing the product, its mechanism of action, and other essential attributes.
Takeaway 6: Consider developing a section on treatment landscape and unmet need
 Although not specified in the guidance for development of a UPD or UUD, describing the treatment landscape for the indication can help the payer fully understand the disease and the unmet need. As such, it may be helpful to include a brief section describing available treatment (or lack thereof).
Although not specified in the guidance for development of a UPD or UUD, describing the treatment landscape for the indication can help the payer fully understand the disease and the unmet need. As such, it may be helpful to include a brief section describing available treatment (or lack thereof).
Additional Tips from RTI Health Solutions’ Global Value Dossier Development Experts
![]()
When determining the appropriate timing for developing a UPD vs. a post-approval AMCP dossier, understand that the two are not distributed to payers at the same time. Upon product approval, the post-approval AMCP dossier will supersede the UPD, which would then no longer be distributed. However, if approval is delayed, a manufacturer may need to continue to update the UPD to communicate new data as it becomes available.
A high-quality UPD can be used as the foundation for the post-approval dossier. Developers can add updated references to the dossier as they become available, thus expanding the dossier organization and content to reflect the AMCP format guidelines for an approved product dossier.
RTI Health Solutions has a team of experienced health outcomes scientists with deep expertise in dossier development. We invite you to learn more about how we can help with your market access needs here. Please reach out if you have any questions.
Download a PDF of our Guidance Summary